Determine Mass Of Ni2+ In Ni Dmg 2
1,8 pDPS(0,95.100)+(0,05.180) = 95+9 = 104 pDPSnow lets say it has 30% crit roll and 30% multiplier roll5.0,3 + 5 = 6,5% overall crit chance(0,935.100)+(0,065.180)= 93,5+11,67 = 105,2and so onThat said, this calculation means nothing since your build will influence it a lot. Hence these factors are not included in wep DPS cacl.Accuracy is another matter and totally depends on your builds accuracyif your build has 50% chance to hit, additional 300 accuracy (in end game) can have a huge impact on your chance to hit and your dps. For instance it can raise your chance to hit to 60% (thats a 20% dps increase)if you have 90% chance to hit, 300 additional accuracy will have a very small impact on your dps. Calculate dps with dmg and rpm online. The more% crit chance u have in tree and crit mulitplier the bigger the impact will base crit chance of the wep have.
- Determine Mass Of Ni2+ In Ni Dmg 2018
- Determine Mass Of Ni2 In Ni Dmg 2 Structure
- Determine Mass Of Ni2+ In Ni Dmg 2015
- Determine Mass Of Ni2+ In Ni Dmg 2016
- Determine Mass Of Ni2 In Ni Dmg 2 Pdf
- Determine Mass Of Ni2 In Ni Dmg 2 Precipitate
Balance the reaction of NiCl2 + DMG + NH4OH = Ni(DMG)2 + NH4Cl + H2O2 using this chemical equation balancer! Calculate the percent nickel present in the ore. Notes: If transparent, colorless needle-like crystals appear in the final nickel dimethylgloxime, or if the results of the nickel content in the ore have a high degree of variance, use the following procedure: 1. Fill each crucible containing the Ni(DMG) 2. Question: A Student Weighed Out 3.5689 G Of Unknown Nickel Salt And Carried Out The Gravimetric Analysis, Following The Procedure In The Lab Manual, In Order To Determine The% Nickel In The Unknown Sample. The Average Masses Of The Two Bis(dimethylglyoximate)nickel(II) Precipitates That Was Filtered Was 0.1570 G.
Determine Mass Of Ni2+ In Ni Dmg 2018
| Names | |
|---|---|
| IUPAC name | |
Other names
| |
| Identifiers | |
| |
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.002.201 |
| EC Number | |
PubChemCID | |
| RTECS number |
|
| UNII | |
CompTox Dashboard(EPA) | |
| |
| |
| Properties | |
| C4H8N2O2 | |
| Molar mass | 116.120 g·mol−1 |
| Appearance | White/Off White Powder |
| Density | 1.37 g/cm3 |
| Melting point | 240 to 241 °C (464 to 466 °F; 513 to 514 K) |
| Boiling point | decomposes |
| low | |
| Structure | |
| 0 | |
| Hazards | |
| Main hazards | Toxic, Skin/Eye Irritant |
| Safety data sheet | External MSDS |
| GHS pictograms | |
| GHS Signal word | Danger |
| H228, H301 | |
| P210, P240, P241, P264, P270, P280, P301+310, P321, P330, P370+378, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
| Hydroxylamine salicylaldoxime | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| verify (what is ?) | |
| Infobox references | |
Dimethylglyoxime is a chemical compound described by the formula CH3C(NOH)C(NOH)CH3. Its abbreviation is dmgH2 for neutral form, and dmgH for anionic form, where H stands for hydrogen. This colourless solid is the dioxime derivative of the diketone butane-2,3-dione (also known as diacetyl). DmgH2 is used in the analysis of palladium or nickel. Its coordination complexes are of theoretical interest as models for enzymes and as catalysts. Many related ligands can be prepared from other diketones, e.g. benzil.
Preparation[edit]
Determine Mass Of Ni2 In Ni Dmg 2 Structure
Dimethylglyoxime can be prepared from butanone first by reaction with ethyl nitrite to give biacetyl monoxime. The second oxime is installed using sodium hydroxylamine monosulfonate:[1]

Complexes[edit]
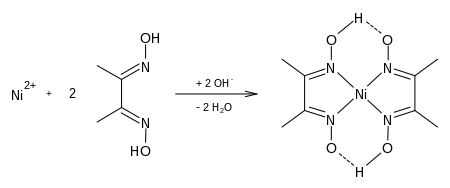
Dimethylglyoxime is used to detect and quantify nickel, which forms the bright red complex nickel bis(dimethylglyoximate) (Ni(dmgH)2). The reaction was discovered by L. A. Chugaev in 1905.[2]
Determine Mass Of Ni2+ In Ni Dmg 2015
Cobalt complexes have also received much attention. In chloro(pyridine)cobaloxime[3] the macrocycle [dmgH]22− mimics the macrocyclic ligand found in vitamin B12.
References[edit]
Determine Mass Of Ni2+ In Ni Dmg 2016
Determine Mass Of Ni2 In Ni Dmg 2 Pdf
- ^Semon, W. L.; Damerell, V. R. (1930). 'Dimethylglyoxime'. Organic Syntheses. 10: 22. doi:10.15227/orgsyn.010.0022.CS1 maint: multiple names: authors list (link)
- ^Lev Tschugaeff (1905). 'Über ein neues, empfindliches Reagens auf Nickel'. Berichte der Deutschen Chemischen Gesellschaft. 38 (3): 2520–2522. doi:10.1002/cber.19050380317.
- ^Girolami, G. S.; Rauchfuss, T.B.; Angelici, R. J. (1999). Synthesis and Technique in Inorganic Chemistry: A Laboratory Manual (3rd ed.). pp. 213–215.
